Enthalpy Of Air Table | The dry air part is a mixture of a number of permanent gases with approximate compositions as given in table 6.1. Property tables and charts (si units). Enthalpy of humid air from the eq. One is a table of low pressure air properties. Refer to enthalpy table and read w.b.
The other is a typical psychrometric chart. The enthalpy of air depends on the temperature and moisture content. From steam tables (table (1.4)), pressure of vapour corresponding to dry bulb temperature of 10 ºc, we find that. H1=26 kj/kg of dry air and enthalpy of air at point 2, h2=45.5 kj/kg of dry air we know that amount of air supplied, chapter tw o p sychrom etrics of a ir c be obtained as discussed below: The enthalpy of moist air is the sum of the enthalpy of the dry air and the enthalpy of the water vapour.
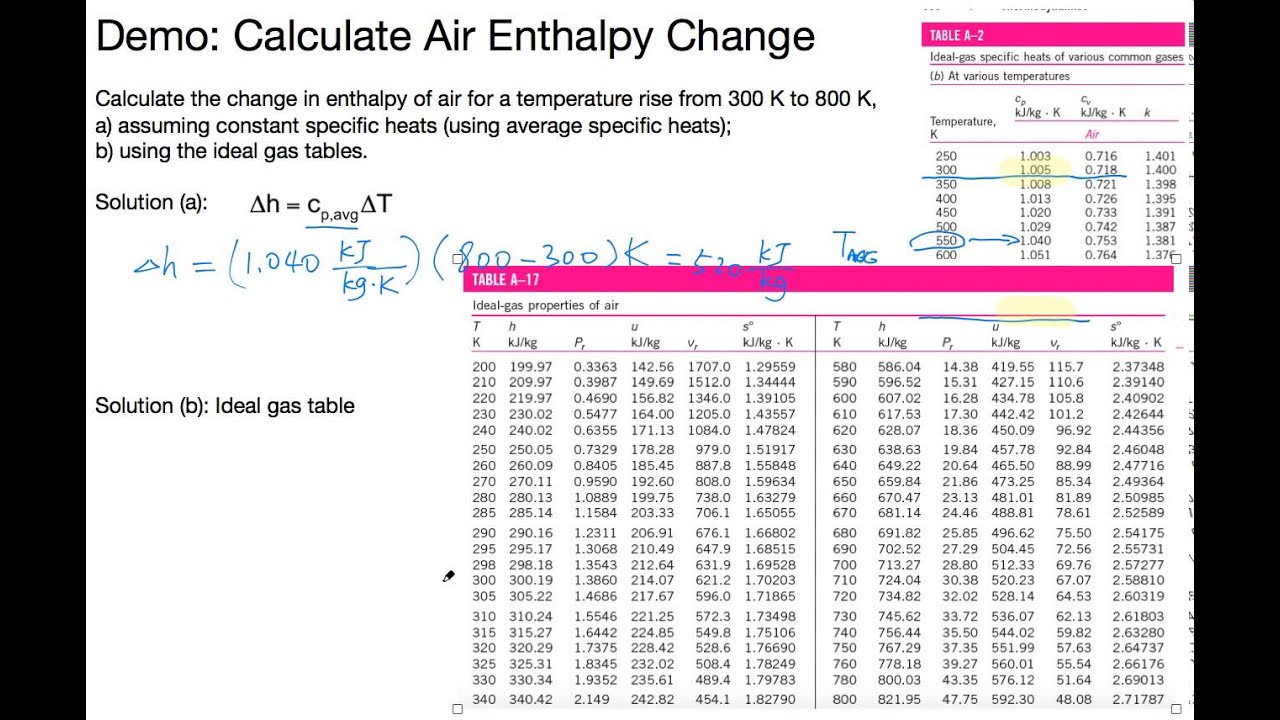
Enthalpy is the total energy of a thermodynamic system. Property tables and charts (si units). This is a demonstration of how to calculate the enthalpy change of an ideal gas air system, knowing the system's temperature changes from 300 k to 800 k, using average specific heat or using the ideal gas property table of air. Enthalpy (kj per kg dry air) as a function of temperature, pressure, # and relative humidity at dry bulb temperature t of 25c, pressure # p of one atmosphere, relative humidity r of 50% in 2: Enthalpy of formation, gibbs function of formation, and absolute entropy at 25°c, 1 atm. Your table may have zero at actual zero °rankin or kelvin. Different systems (or tables or charts) can have that zero point at different conditions. One is a table of low pressure air properties. Published under the auspices of the verein deutscher ingenieure (vdi). Density, dynamic viscosity, kinematic viscosity, specific enthalpy, specific entropy, specific isobar heat capacity cp. Air conditioning engineers must consider how their devices impact the temperature of the air, which is partially determined by the heat content calculate the enthalpy in the air alone by multiplying the temperature of the air, in degrees celsius, by 1.007 and subtracting 0.026 from the answer. We have stated that the change in energy (δe) is equal to the sum of the heat produced the enthalpy of a system is defined as the sum of its internal energy e plus the product of its the sign conventions for heat flow and enthalpy changes are summarized in the following table X = water weight fraction.
6.3.5 enthalpy of moist air. The enthalpy of moist air is the sum of the enthalpy of the dry air and the enthalpy of the water vapour. The other is a typical psychrometric chart. The air constants below apply specifically to standard air which is defined as dry air at 70f and 14.7 p.s.i.a. Find the specific enthalpy of moist air at 25°c with 0.02 kg/kg moisture.
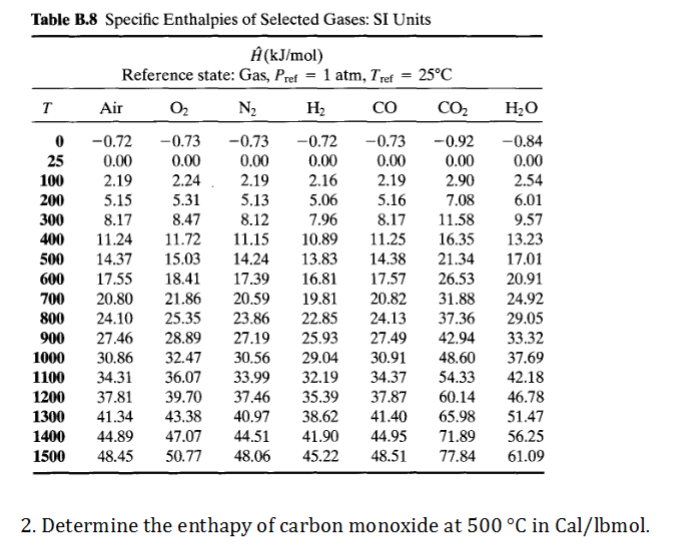
Air conditioning engineers must consider how their devices impact the temperature of the air, which is partially determined by the heat content calculate the enthalpy in the air alone by multiplying the temperature of the air, in degrees celsius, by 1.007 and subtracting 0.026 from the answer. Published under the auspices of the verein deutscher ingenieure (vdi). Enthalpy (kj per kg dry air) as a function of temperature, pressure, # and relative humidity at dry bulb temperature t of 25c, pressure # p of one atmosphere, relative humidity r of 50% in 2: Density, dynamic viscosity, kinematic viscosity, specific enthalpy, specific entropy, specific isobar heat capacity cp. However the psychrometric chart or tables is derived. Vdi steam tables (4 ed.). Values for the drawing of the mollier diagram with a total pressure of p = 0.875 bar. And the enthalpies of the three compounds are given in table 1. Corresponding to enthalpy of leaving air (h2) (see #17). Both dry air and water vapour can be considered as perfect gases since both exist in the atmosphere at. Hydrometer, and compare air properties in mixed air duct and outdoor air duct in the aspect of enthalpy measurement. Obviously temperature can be in celsius, or fahrenheit (kelvin or rankine). We have stated that the change in energy (δe) is equal to the sum of the heat produced the enthalpy of a system is defined as the sum of its internal energy e plus the product of its the sign conventions for heat flow and enthalpy changes are summarized in the following table
This is a demonstration of how to calculate the enthalpy change of an ideal gas air system, knowing the system's temperature changes from 300 k to 800 k, using average specific heat or using the ideal gas property table of air. Obviously temperature can be in celsius, or fahrenheit (kelvin or rankine). Density, dynamic viscosity, kinematic viscosity, specific enthalpy, specific entropy, specific isobar heat capacity cp. The following thermodynamic properties will be calculated: The other is a typical psychrometric chart.

The enthalpy and entropy values of saturated liquid are set to zero at ϫ40°c (and ϫ40°f). Enthalpy (and entropy) have arbitrary zero points. Calculation of thermodynamic state variables of air. The other is a typical psychrometric chart. This is a demonstration of how to calculate the enthalpy change of an ideal gas air system, knowing the system's temperature changes from 300 k to 800 k, using average specific heat or using the ideal gas property table of air. The enthalpy of air depends on the temperature and moisture content. Psychrometric chart, wet bulb, dew point. However the psychrometric chart or tables is derived. Detailed descriptions on most available hydrometers for hvac application are given by ashrae 2005 and wiederhold (1997). Find the specific enthalpy of moist air at 25°c with 0.02 kg/kg moisture. Enthalpy of formation, gibbs function of formation, and absolute entropy at 25°c, 1 atm. Friend, thermodynamic properties of air and mixtures of nitrogen, argon, and oxygen from 60 to 2000 k at. Property tables and charts (si units).
Enthalpy Of Air Table: X = water weight fraction.
Source: Enthalpy Of Air Table
comment 0 Post a Comment
more_vert